 Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment.
Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment.
Ingredients:
- Knowledge Base: Gather all relevant information about the accreditation process. Understand the standards, criteria, and expectations set by the accrediting body.
- Documentation: Ensure all necessary documentation is in order. This may include policies, procedures, and evidence of compliance. Proper documentation is the foundation of a successful accreditation assessment.
- Team Collaboration & Training: Establish a cohesive team. Assign responsibilities to team members based on their expertise. Ensure that your team is well-trained and up to date on relevant skills.
- Action Plan: Develop an action plan based on the findings from mock assessments. Your organization should strive for continuous enhancement of processes and procedures.
Instructions:
- Start Early: Begin preparations well in advance of the assessment date. Give yourself ample time to address any unforeseen challenges.
- Gather Information & Documentation: Collect all necessary information about the accreditation process from your chosen accreditation body. Understand the expectations and requirements thoroughly. Ensure all required documentation is complete and organized, which is crucial for a strong foundation during the assessment.
- Develop an Action Plan: Develop an action plan based on the lessons learned from mock assessments.
- Foster Team Collaboration: Assign roles and responsibilities to team members based on their strengths.
- Invest in Training and Development: Devote time for training and development to enhance the skills of your team. A well-prepared team is essential for accreditation success.
- Review and Reflect: Review your preparations, reflect on the process, and make any necessary adjustments. Ensure everything is in order for the assessment.
By following this step-by-step recipe, you are well on your way to preparing for a successful accreditation assessment. Bon appétit to your accreditation journey!
 In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).
In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).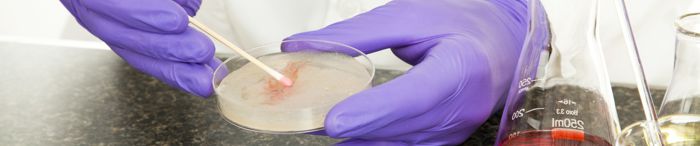 Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted
Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted  In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories.
In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories. Perry Johnson Laboratory Accreditation, Inc. (PJLA) joyfully welcomed assessors back to Troy, Michigan, for our first in-person Annual Training since December 2019. The gathering, held on December 7th and 8th, marked a pivotal moment, fostering an atmosphere for teamwork and professional growth.
Perry Johnson Laboratory Accreditation, Inc. (PJLA) joyfully welcomed assessors back to Troy, Michigan, for our first in-person Annual Training since December 2019. The gathering, held on December 7th and 8th, marked a pivotal moment, fostering an atmosphere for teamwork and professional growth. In the constantly evolving landscape of the laboratory industry, staying attuned to current trends and foreseeing future developments is paramount for success. Today, we’ll explore five prominent shifts shaping the industry and how they impact the dynamics of hospital-based laboratories.
In the constantly evolving landscape of the laboratory industry, staying attuned to current trends and foreseeing future developments is paramount for success. Today, we’ll explore five prominent shifts shaping the industry and how they impact the dynamics of hospital-based laboratories. Call For Presenters: TNI Hosting PFAS Virtual Workshop
Call For Presenters: TNI Hosting PFAS Virtual Workshop The holiday season is a time for joy, togetherness, and of course, delicious meals shared with loved ones. As you embark on creating culinary delights, it is crucial to prioritize safety in the kitchen. Here are some tips to keep in mind for a safe and stress-free cooking experience during the season.
The holiday season is a time for joy, togetherness, and of course, delicious meals shared with loved ones. As you embark on creating culinary delights, it is crucial to prioritize safety in the kitchen. Here are some tips to keep in mind for a safe and stress-free cooking experience during the season. The International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC) held their much-anticipated IAF-ILAC Annual Joint Meetings in Montreal, Canada, from November 6th to 15th, 2023. This event brought together key industry leaders, experts, and stakeholders from across the globe to discuss and strategize on matters pivotal to accreditation and conformity assessment. Among the attendees was Tracy Szerszen, President and Matthew Sica, Technical Program Manager. Their presence marked PJLA’s commitment to staying at the forefront of industry standards and best practices.
The International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC) held their much-anticipated IAF-ILAC Annual Joint Meetings in Montreal, Canada, from November 6th to 15th, 2023. This event brought together key industry leaders, experts, and stakeholders from across the globe to discuss and strategize on matters pivotal to accreditation and conformity assessment. Among the attendees was Tracy Szerszen, President and Matthew Sica, Technical Program Manager. Their presence marked PJLA’s commitment to staying at the forefront of industry standards and best practices. As we celebrate World Quality Week from November 6-10, 2023, the spotlight is on a theme that resonates across industries: “Quality: realizing your competitive potential.” World Quality Week serves as an annual reminder to organizations worldwide about the importance of quality in their operations.
As we celebrate World Quality Week from November 6-10, 2023, the spotlight is on a theme that resonates across industries: “Quality: realizing your competitive potential.” World Quality Week serves as an annual reminder to organizations worldwide about the importance of quality in their operations.