 Measurement Uncertainty refers to the doubt or range of values associated with a measurement result. It’s crucial for understanding the reliability of measurements. One approach that is used by calibration laboratories, and may be used for testing laboratories, includes these steps:
Measurement Uncertainty refers to the doubt or range of values associated with a measurement result. It’s crucial for understanding the reliability of measurements. One approach that is used by calibration laboratories, and may be used for testing laboratories, includes these steps:
- Identify the Measurement Process: Define the measurement process, including the instrument used, the quantity being measured, the measurement procedure, and any relevant environmental factors.
- List Sources of Uncertainty: Identify all potential sources of uncertainty that can affect the measurement result. These can include instrument limitations, calibration errors, environmental conditions, operator skill, etc.
- Classify Uncertainties: Categorize uncertainties into two main types: Type A and Type B.
- Type A: These are evaluated statistically based on a series of repeated measurements. Calculate the standard deviation or standard error of the mean to estimate uncertainty due to random fluctuations.
- Type B: These are assessed using other information sources like calibration certificates, manufacturer specifications, expert opinions, etc. They’re typically evaluated using probability distributions.
- Quantify Type A Uncertainties: Perform multiple measurements of the same quantity under similar conditions. Calculate the mean and standard deviation of these measurements. The standard deviation represents the Type A uncertainty.
- Quantify Type B Uncertainties: For each identified source of uncertainty, gather relevant data or information. Estimate the uncertainty contribution from each source using appropriate statistical methods or available information.
- Combine Uncertainties: Combine Type A and Type B uncertainties to calculate the combined standard uncertainty. This is often done using the root-sum-square (RSS) method. Square each uncertainty component, sum them up, and take the square root of the sum.
- Coverage Factor: Introduce a coverage factor (usually denoted as k) to account for the confidence level. For a Gaussian distribution (which is often assumed), a coverage factor of 2 corresponds to a 95% confidence level.
- Expanded Uncertainty: Multiply the combined standard uncertainty by the coverage factor to obtain the expanded uncertainty. This provides a range within which the true value is likely to lie.
- Report Uncertainty: Present the measurement result along with the expanded uncertainty. This communicates the reliability of the measurement to others.
- Iterative Process: Measurement uncertainty assessment is not a one-time task. It should be periodically reviewed and updated as new information or data becomes available, or if the measurement process changes.
- Documentation: Keep comprehensive records of the measurement process, sources of uncertainty, calculations, and any assumptions made during the assessment. This documentation is crucial for traceability and repeatability.
Testing laboratories often find this approach is not practical. Testing laboratories may choose to use a variety of data-driven approaches using laboratory data from calibrations, quality controls, or validation.
Some of the common approaches using laboratory data include:
- NORDTEST NT TR 537 – “Handbook for calculation of measurement uncertainty in environmental laboratories”
- ISO 21748 – “Guidance for the use of repeatability, reproducibility and trueness estimates in measurement uncertainty estimation”
- ISO 11352 – “Water quality – Estimation of measurement uncertainty based on validation and quality control data”
There are two points for testing laboratories to note: 1) These approaches do not identify the major sources of error, as required by the standard and 2) That single value may not define the uncertainty of a method when using one of these approaches. To address the first point, consider listing or using a fishbone diagram to identify the major sources or error. The considerations in addressing the second include the evaluating the impact over the range of the test and the understanding the impacts of different sample preparations of the items or matrices involved.
Remember that measurement uncertainty assessment is both a scientific and mathematical process. It requires a thorough understanding of the measurement process, statistical methods, and relevant data sources.
For more information, please email [email protected] or call (248) 519-2603.
Want more information on Measurement Uncertainty? Join PJLA and Dr. Susan Audino for a complimentary webinar on Tuesday, February 20, 2024 – 1:00pm ET. She will be presenting Measurement Uncertainty and Decision Rules: Why Do They Matter?
To download this QuickStart Guide to Measurement Uncertainty, please CLICK HERE.
 Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment.
Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment. In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).
In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).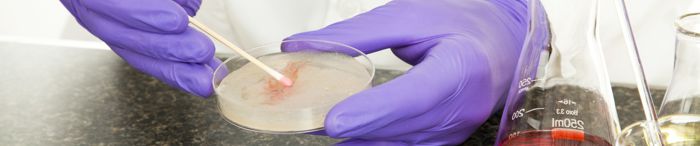 Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted
Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted  In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories.
In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories. Perry Johnson Laboratory Accreditation, Inc. (PJLA) joyfully welcomed assessors back to Troy, Michigan, for our first in-person Annual Training since December 2019. The gathering, held on December 7th and 8th, marked a pivotal moment, fostering an atmosphere for teamwork and professional growth.
Perry Johnson Laboratory Accreditation, Inc. (PJLA) joyfully welcomed assessors back to Troy, Michigan, for our first in-person Annual Training since December 2019. The gathering, held on December 7th and 8th, marked a pivotal moment, fostering an atmosphere for teamwork and professional growth. In the constantly evolving landscape of the laboratory industry, staying attuned to current trends and foreseeing future developments is paramount for success. Today, we’ll explore five prominent shifts shaping the industry and how they impact the dynamics of hospital-based laboratories.
In the constantly evolving landscape of the laboratory industry, staying attuned to current trends and foreseeing future developments is paramount for success. Today, we’ll explore five prominent shifts shaping the industry and how they impact the dynamics of hospital-based laboratories. Call For Presenters: TNI Hosting PFAS Virtual Workshop
Call For Presenters: TNI Hosting PFAS Virtual Workshop The holiday season is a time for joy, togetherness, and of course, delicious meals shared with loved ones. As you embark on creating culinary delights, it is crucial to prioritize safety in the kitchen. Here are some tips to keep in mind for a safe and stress-free cooking experience during the season.
The holiday season is a time for joy, togetherness, and of course, delicious meals shared with loved ones. As you embark on creating culinary delights, it is crucial to prioritize safety in the kitchen. Here are some tips to keep in mind for a safe and stress-free cooking experience during the season. The International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC) held their much-anticipated IAF-ILAC Annual Joint Meetings in Montreal, Canada, from November 6th to 15th, 2023. This event brought together key industry leaders, experts, and stakeholders from across the globe to discuss and strategize on matters pivotal to accreditation and conformity assessment. Among the attendees was Tracy Szerszen, President and Matthew Sica, Technical Program Manager. Their presence marked PJLA’s commitment to staying at the forefront of industry standards and best practices.
The International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC) held their much-anticipated IAF-ILAC Annual Joint Meetings in Montreal, Canada, from November 6th to 15th, 2023. This event brought together key industry leaders, experts, and stakeholders from across the globe to discuss and strategize on matters pivotal to accreditation and conformity assessment. Among the attendees was Tracy Szerszen, President and Matthew Sica, Technical Program Manager. Their presence marked PJLA’s commitment to staying at the forefront of industry standards and best practices.