 Dear Perry Johnson Family,
Dear Perry Johnson Family,
It is with heavy hearts that we share the news of the passing of our dear friend and esteemed colleague, Ray Wegner. Ray dedicated an incredible 26 years of his life to Perry Johnson Companies, leaving an indelible mark on our organization and the lives of those fortunate enough to know him.
Ray served as a beacon of commitment and passion, working tirelessly as a Regional Sales Manager in our Chicago office. His unwavering dedication to excellence, coupled with his warm and friendly demeanor, made him an integral part of our company’s success and a beloved member of our close-knit family.
Beyond his professional accomplishments, Ray was a true friend to many, always ready with a smile, a kind word, and a helping hand. His positive attitude, strong work ethic, and infectious enthusiasm inspired everyone around him, creating a workplace culture that reflected his values of teamwork and mutual support.
Our thoughts and prayers are with Ray’s family during this difficult time, and we extend our heartfelt condolences to them. To send flowers to the family or plant a tree in memory of Raymond Joseph Wegner, please Click Here.
May he rest in peace!

 In the dynamic world of testing and analysis, the choice between an accredited and a non-accredited laboratory can significantly impact the reliability and credibility of results. Perry Johnson Laboratory Accreditation, Inc. (PJLA) stands as a beacon of quality assurance, and we’re here to shed light on the undeniable benefits of choosing an accredited laboratory over a non-accredited one.
In the dynamic world of testing and analysis, the choice between an accredited and a non-accredited laboratory can significantly impact the reliability and credibility of results. Perry Johnson Laboratory Accreditation, Inc. (PJLA) stands as a beacon of quality assurance, and we’re here to shed light on the undeniable benefits of choosing an accredited laboratory over a non-accredited one. With a robust network of international offices, PJLA is uniquely positioned to provide comprehensive assistance to organizations worldwide. Our global presence ensures that clients can access accreditation services tailored to their specific needs, regardless of geographical location. The diverse expertise of our team, coupled with in-depth understanding of regional regulatory frameworks, enables seamless navigation through the accreditation process.
With a robust network of international offices, PJLA is uniquely positioned to provide comprehensive assistance to organizations worldwide. Our global presence ensures that clients can access accreditation services tailored to their specific needs, regardless of geographical location. The diverse expertise of our team, coupled with in-depth understanding of regional regulatory frameworks, enables seamless navigation through the accreditation process. In the intricate world of accreditation, where precision is paramount and accuracy is everything, the pursuit of excellence is never-ending. To stand out in this competitive landscape, companies must go above and beyond the norm, and accreditation is the key that unlocks the doors to success. Perry Johnson Laboratory Accreditation, Inc. (PJLA) emerges as the guiding light on your accreditation journey, offering a comprehensive range of accreditation services, including Proficiency Testing Provider Accreditation, Inspection Body Accreditation, Laboratory Accreditation, and
In the intricate world of accreditation, where precision is paramount and accuracy is everything, the pursuit of excellence is never-ending. To stand out in this competitive landscape, companies must go above and beyond the norm, and accreditation is the key that unlocks the doors to success. Perry Johnson Laboratory Accreditation, Inc. (PJLA) emerges as the guiding light on your accreditation journey, offering a comprehensive range of accreditation services, including Proficiency Testing Provider Accreditation, Inspection Body Accreditation, Laboratory Accreditation, and  PJLA has gathered data from the ISO/IEC 17025 assessments held in 2023 for both testing and calibration laboratories. Here were the Top Ten Common Findings observed throughout the year:
PJLA has gathered data from the ISO/IEC 17025 assessments held in 2023 for both testing and calibration laboratories. Here were the Top Ten Common Findings observed throughout the year: Measurement Uncertainty refers to the doubt or range of values associated with a measurement result. It’s crucial for understanding the reliability of measurements. One approach that is used by calibration laboratories, and may be used for testing laboratories, includes these steps:
Measurement Uncertainty refers to the doubt or range of values associated with a measurement result. It’s crucial for understanding the reliability of measurements. One approach that is used by calibration laboratories, and may be used for testing laboratories, includes these steps: Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment.
Embarking on your first accreditation assessment can be a daunting task, much like stepping into the kitchen to prepare a new and complex dish. However, with the right ingredients and a well-thought-out recipe, success is possible. In this guide, we will outline the step-by-step recipe to ensure smooth and successful preparation for your first accreditation assessment. In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).
In the dynamic world of accreditation, the significance of outstanding customer service cannot be overstated. A stellar example of this is Sean Donnellan, a National Project Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA).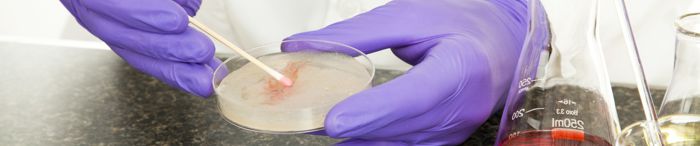 Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted
Matthew Sica, a Technical Program Manager at Perry Johnson Laboratory Accreditation, Inc. (PJLA), recently hosted  In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories.
In the realm of food safety, the discourse surrounding Rapid Microbiological Methods (RMM) has been both progressive and contentious. The debate centers on whether these methods, once hailed for their efficiency in pathogen detection, remain indispensable. The landscape has transformed significantly over the years, with an increasing reliance on outsourcing pathogen samples due to safety concerns inherent in in-plant food laboratories.